Original Article
Year: 2020 |Volume: 1 | Issue: 1 |Pages: 9-13
Physicochemical Standardization of Lekhan Basti
About Author
Correspondence Address:
Dr. Swapnil Auti, Associate Professor, Faculty of Indian medical system, SGT university. [Email- swapn.punarvasu19@gmail.com Mob-8446318328]
Date of Acceptance: 2020-04-16
Date of Publication:2020-05-19
Article-ID:IJIM_2_05_20 http://ijim.co.in
Source of Support: None
Conflict of Interest: None
How To Cite This Article: Auti SS, Thakar AB, Shukla VJ. Physicochemical Standardization of Lekhan Basti. Int. J Ind. Med. 2020;1(1):9-13
Abstract
Ayurvedic Panchakarma formulations especially Basti preparations are highly specific according to the disease conditions. Moreover, classical texts have specified quantities of each content as per the Doshik predominance. Thus final prepared product is having a specific physicochemical characteristic which may alter with the bio availability of the active principles, functional properties, retention time etc. of the Basti. Lekhan Basti is one such formulation advised specially in Medoroga. Thus with view to standardised this formulation physicochemical analysis of Lekhan Basti was done. According to Standardized physicochemical data of Lekhan Basti its Specific Gravity, Density, Refractive Index, pH, Total Solid Contents, Viscosity and Tannin contents were 1.14, 1.14 g/ml, 1.408, 4.0, 59% w/v, 4.12 and 4.3% w/v respectively.
Keywords: Lekhan Basti, Standardization, Medoroga, Panchakarma
Introduction
Panchakarma has evolved as a promising cure in many diseases. The world is looking towards the Ayurveda with great hope and so many queries especially about quality standards of Ayurvedic formulations. So many Basti preparations are practiced with variety of differences as per the understanding of the practitioners & available classical references. In this situation standardization of these formulations is highly essential to assure the reliability especially in terms of quality, purity, efficacy and safety of the formulation.
Contents of the Basti formulation have been specified by all Acharyas with a fixed dose as per the Doshik dominance or depending upon the drugs used, in various scattered references. Today Ayurvedic science is spreading its wings all over the world where the drug prepations of this system has become the center of global interest. Hence it has become the need of time to standardise Panchakarma formulations in terms of physico-chemical parameters for a quality assurance. Also Drug absorption & further kinetics of its components depends upon some physicochemical properties. With this background, present analytical study on Lekhan Basti was undertaken with objective to analyze the Lekhan Basti by using different physicochemical parameters.
Materials And Methods:
Lekhan Basti[1] was prepared without altering the classical methods and by using authenticated raw material as follows,
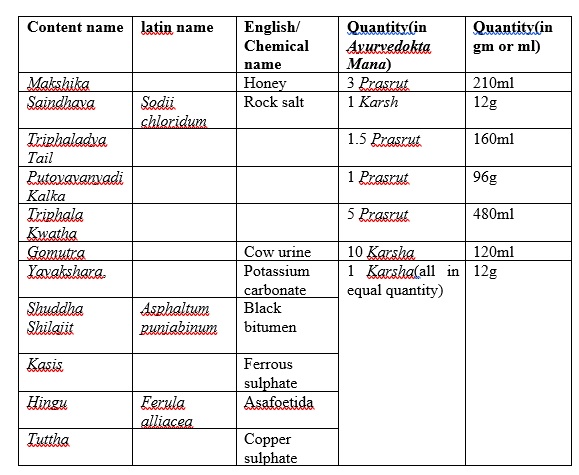
Table 1: Contents of Lekhan Basti
Contents of Lekhan Basti
Twelve Prasruta is standard dose for Madhyam Ayu patient, it generally denotes volumetric considerations and thus twelve Prasruta is a fixed quantity if taken every ingredient as per volume. But as per the trend & tradition among now a days practitioners, weight of the ingredients are considered as a standard criteria. But this concept has a practical problem i.e. all the ingredients of the basti in equal volumes differs in their weights due to difference in their specific gravities. Thus contents taken in Prasruta Mana by converting them to grams should be done considering their specific gravities then only the weight of the Basti prepared finally will be equal to the weight calculated by converting Prasruta to grams. For this purpose in present study quantities of ingredients in liquid form were determined in grams as follows by considering the specific gravities of the basic component of the formulation:
Principle to convert Grams into Millilitres: Divide the number of grams by the specific gravity of the substance, to obtain the volume in millilitres. [2]
For Honey:
Specific gravity of honey -1.3935[3]
Hence, 1g of honey/1.3935= 0.72 ml
For Lekhan Basti the dose decided for honey was 3 Prasruta i.e. 288 g,
Therefore, in millilitres it come to be,
288 gram of Honey ×0.72=207.36 ml
Therefore, approximately 210ml was the quantity taken for 3 Prasruta of honey in present study.
For Triphaladya Tail :
Til Tail is the basic ingredient of Triphaladya Tail hence to calculate an approximate value in mililiters, the specific gravity of Til Tail is considered for the required weight of Triphaladya Tail.
Specific gravity of Sesame oil-0.922[4]
Hence,
1g of Sesame oil/0.922= 1.08 ml
For Lekhan Basti the dose decided for Sneha was 1.5 Prasruta i.e. 144 g,
Therefore, in millilitres it come to be,
144g of oil ×1.08=155.52 ml
Therefore, approximately 160ml was the quantity taken for 1.5 Prasruta of Triphaladya Tail in present study.
For Gomutra (cow urine):
Specific gravity of Gomutra -1.045[5]
Hence, 1g of Gomutra/1.045= 0.96 ml
For Lekhan Basti the dose decided for Gomutra was 10 Karsha i.e. 120 g,
Therefore, in millilitres it come to be,
120 gram of Gomutra ×0.96=115.2 ml
Therefore, approximately 120ml was the quantity taken for 10 Karsha of Gomutra in present study.
However for Kwatha, The water is the basic component. Though specific gravity of water is different than Kwatha but the difference would be negligible in terms of approximation. Hence the conversion is considered same as for water 1gm=1ml.
Thus, by calculating this way & taking ingredients the final prepared Dvadashaprasruta Basti comes to be of 1000 ml.
The parameters used for the analysis has been mentioned below:
1. Physico-chemical parameters:
- Specific gravity
- Density
- Refractive index
- pH
- Total solid contents
- Viscosity
- Tannin contents
Specific gravity[6]:
A clean and dry 25 ml capacity Pycnometer was taken and its weight was noted. It was filled with the sample, cleaned properly from outside and the weight was taken at 40°C. Then it was cleaned, rinsed and filled with distilled water, dried from outside and the weight was noted at 40°C. The weight of sample and distilled water was calculated. Then the Specific gravity was determined by dividing the weight of the sample by the weight of the water. Density is calculated by dividing weight of the sample by volume of the sample taken.
Refractive Index[7]:
Refractive index of a substance varies with temperature. Hence, temperature is to be noted while determining R.I. The R.I. of different samples was measured by using Abbe’s Refracto-meter at 40°C. The temperature was maintained at 40°C by circulating warm water.
Determination of pH[8]:
25 ml of Lekhan Basti was taken in a beaker. pH paper is dipped in the sample & slightly washed with distilled water to note the colour change. The observed colour is matched with standard scale.
Total solid contents[9]:
25 ml of Lekhan Basti sample was taken in a previously dried and weighed evaporating dish, evaporated on water bath and further dried in an oven at 110°C till constant weight. From the weight of the residue obtained the percentage of total solid content in the sample was determined and expressed as percentage w/v.
Determination of Viscosity[10]:
Lekhan Basti is poured in the viscometer so that, it remains below the level marked as ‘A’. Then the Lekhan Basti is sucked above with mouth beyond level ‘B’. Then with pressure of finger it is maintained exactly at level ‘B’. Pressure is released completely & time taken by solution to reach up to level ‘A’ is recorded by stopwatch. Same procedure is repeated trice to get an average value. Same procedure is repeated by using distilled water instead of Lekhan Basti.
Viscosity was calculated using following equation,
Viscosity= Average time taken by sample× Density of the samplz/ Average Time taken by Distilled water× Density of Distilled water
Determination of Total Tannins[11]:
Total tannins of the Lekhan Basti were determined by using Lowenthal's method improved by Councler, Shroeder, and Procter[12]
Results:
Table 2: Analytical data of Lekhan Basti
|
Parameter studied |
Values obtained |
|
1.14 |
|
1.14 g/ml |
|
1.408 |
|
4.0 |
|
59% w/v |
|
4.12 |
|
4.3% w/v |
Discussion
Physicochemical standardization of Lekhan Basti is required to reproduce the same clinical or experimental data in every experiment. Moreover parameters like absorption of active ingredients in Basti, selective receptor stimulation, effect on microbial flora etc. depends largely on physicochemical characteristics thus it becomes more important to know what exactly the analytical data is. The density of a solution is the sum of mass (massic) concentrations of the components of that solution. Density defined in a qualitative manner as the measure of the relative "heaviness" of objects with a constant volume. [13] Lekhan Basti is found to have relatively high specific gravity & more density due to the effect of combined specific gravities & densities of its contents. Viscosity describes a fluid's internal resistance to flow and may be thought of as a measure of fluid friction. Refractive index is a fundamental physical property of a substance, it is often used to identify a particular substance, confirm its purity, or measure its concentration. Refractive index is used to measure solids liquids, and gases. Most commonly it is used to measure the concentration of a solute in an aqueous solution. [14]
Total solid contents represents % by weight of the whole which is non volatile at a definite temperature in an open atmosphere. [15] Lekhan Basti is a mixture of many compounds. Its high solid content (59% w/v) & more viscosity (4.12) may be attributed to the incorporation of solid substances like Ushakadi Gana Prakshepa & honey (Viscosity of honey[16] 2-10) respectively which adds a maximum part. As many substances are in the solute form constitutes the final product of Basti it has low refractive index of 1.408. Tannin content of the Lekhan Basti is because of Triphala as it is reach in tannins (>30%).[17] pH of the Basti is nearly weak acidic,[18] though some substances incorporated in the Basti are having basic pH(e.g cow urine pH=7.4).[19] This is primarily because of reach quantity of Triphala Kashaya in the Basti the contents of which are acidic (pH of Emblica officinalis[20] =2.5-3.8, Terminalia chebula[21] =2.5-5.5). Weakly acidic drugs are more readily absorbed from an acid medium. The pH in the colon varies between 5.5 and 7 (slightly acidic to neutral), [22] this weak acidic media in colon may facilitate the drug absorption.
Conclusion
According to Standardized physicochemical data of Lekhan Basti its Specific Gravity is 1.14, Density is 1.14 g/ml, Refractive Index is 1.408, pH is 4.0, Total Solid Contents are 59% w/v, Viscosity is 4.12 and Tannin contents are 4.3% w/v.
References
value="
- Shastri A, editor, Sushruta, Nagarjuna, Sushruta Smhita, Sushruta Chikitsa sthana 38/82, 1st Edition, Chaukhamba Sanskrit Pratishthana Varanasi, 2005; 545
- http://www.testdiet.com/conversions.htm retrieved 2011-3-1
- http://www.123foodscience.com/Food_analysis/honey/food+analysis+honey+analysis+determination_of_specificgravity.php retrieved2011-3-1
- The Wealth of India : A Dictionary of Indian Raw Materials and Industrial Products
- Reece W.O., Water Balance and Excretion, in Dukes’ Physiology of Domestic Animals, 11th ed., Swenson M.J. and Reece W.O. Eds. Copyright©1993 by Cornell University.
- Density definition in Oil Gas Glossary". Oilgasglossary.com. http://oilgasglossary.com/density.html. Retrieved 2010-09-14.
- www.thephysicsteacher.ie/LC%20Physics/.../4.%20Refraction.doc retrived 2011-02-13
- http://en.wikipedia.org/wiki/PH retrived 2011-02-13
- Chemical Analysis and Testing Task Laboratory Analytical Procedure LAP-012 by Tina Ehrman Issue Date: 7/5/94
- http://www.sciencebyjones.com/viscosity_overview.htm retrived 2011-02-13
- Councler and Schroeder, Ztsch. anal. Chem. 25, 121. Procter, .Journ. Soc. Chem. Ind., 3, 82.
- Councler and Schroeder, Ztsch. anal. Chem. 25, 121. Procter, .Journ. Soc. Chem. Ind., 3, 82.
- http://www.elmhurst.edu/~chm/vchembook/120Adensity.html retrieved 2011-03-05
- http://en.wikipedia.org/wiki/Refractive_index retrieved 2011-03-05
- http://chemistryprojects.blogspot.com/2010/01/define-total-solid-content-tsc-of-latex.html retrieved 2011-03-05
- http://en.wikipedia.org/wiki/Viscosity retrieved 2011-03-05
- http://www.naturalremedy.com/triphala.htm retrieved 2011-03-05
- http://wiki.answers.com/Q/Is_pH_4_an_weak_acid retrieved 2011-03-05
- cat.inist.fr/?aModele=afficheN&cpsidt=17999826 retrieved 2011-03-05
- www.allianceingredients.com/pdfdocs/EMBLICA.pdf retrieved 2011-03-05
- www.naturalremedy.com › Home › Human Health Care retrieved 2011-03-05
- en.wikipedia.org/wiki/Colon_(anatomy) retrieved 2011-03-05
"

