Original Article
Year: 2020 |Volume: 1 | Issue: 9 |Pages: 362-371
ANALYTICAL STUDY OF SWARNAMAKSHIKA BHASMA AS PER RASATARANGINI
About Author
Correspondence Address:
Dr. Rajni Bhardwaj- Assistant Professor, Department of Rasashastra & Bhaishajya Kalpana. Desh Bhagat Ayurvedic College & Hospital Desh Bhagat University, Mandi Gobindgarh (Punjab). contact No. 9815145010
Date of Acceptance: 2020-12-18
Date of Publication:2020-11-07
Article-ID:IJIM_46_01_20 http://ijim.co.in
Source of Support: NIL
Conflict of Interest: NIL
How To Cite This Article: Rajni Bhardwaj et al. Analytical Study of Swarnamakshika Bhasma As Per Rasatarangini Int. J Ind. Med. 2020;1(9):362-371
Abstract
Today’s era of globalisation everything regarding any research should be explained in a Universal language. Lack of use of scientific approach is one among the important factors hindering the globalisation of Ayurveda. Though traditional methods of analysis for any prepared Ayurvedic medicine, holds unique approach and have been time-tested but in the present scenario there is a necessity of understanding the drug based on modern technology of analysis as well. In Ayurvedic systems of medicines rasa Chikitsa recommends the use of metal and minerals in the form of Bhasma and kupipakwa Rasayanas because when used in proper doses, they are reported to be free from toxic effect on body tissues. Swarnamakshika is known as Saklamyaghan (cures all diseases) and Prana of Parada. In this study analytical study of Ashudha Swarnamakshika (ASM), and prepared classical Swarnamakshika Bhasma (SMB) was carried out according to both classical as well as modern parameters. Physicochemical analysis of Swarnamakshika Bhasma was done after first, fifth and tenth puta.
Keywords: Swarnamakshika Bhasma(SMB), Ashudha Swarnamakshika(ASM), Kupipakva Rasayana
Introduction
Standardization is a tool for confirming the quality and is used to define all procedures which are taken during manufacturing process and its quality control. Ayurvedic granthas have described antient methods of quality control of Bhasmas through different parameters like Rekhapurntva, Nischandratva, Apunarbhava, Varitara, Nirutha, etc.1 To achieve a specific acceptable standard Bhasma. These analytical methods for Bhasma could be used as standards for ensuring quality and reproducibility standards of medicines. Ancient literature use of Swarnamakshika was mentioned by various acharyas. Acharya Charaka, maintained Parada treated by Swarnamakshika and Gandhaka is recommended in treatment of kushta chikitsa.2 Swarnamakshika yoga which contains mainly Swarnamakshika along with other minerals and herbs is recommended in Pandu roga chikitsa.3 In context of Madhumeha Chikitsa, taste, luster and therapeutic properties of Swarnamakshika are described in Shushruta Samhita.4 Considering Swarnamakshika as one of best Rasayana dravya by Vridha Vagbhata in Ashtanga Samgraha-Uttara tantra. Mythological origin, occurrence, synonyms and different varieties and therapeutical qualities are available in detail.5 Rasaratmasamucchaya (13th cent) gave detailed description of Swarnamakshika regarding its synonyms, qualities, occurrence, shodhana, marana & satvapatana procedures. He has grouped it under Maharasa varga.6 Analysis and standardization of Rasaushadhies is knowledge of analytical chemistry is very much essential. Analytical study is a tool to obtain evidence about the qualitative and quantitative composition of substances and chemical species, i.e to find out what a substance elementary compostion, Analytical study has its influence on pharmaceutical research, quality control and in clinical analysis. standerd chemical and instrumental test were working to detect abnormal and normal component. To established the new drug or prove the efficacy of old drug pharmaceutical studies are carried out for proving therapeutic value of a drug before the drug is available to the general population.
Methodology
The material and method used in preparation of Swarnamakshik bhasma were discussed under this heading. Collection of raw material was done from Sri Herbacia Biotech, Amritsar, and proper authentication of raw material was done from Drug Testing Lab. Patiala. Shodhana of Swarnamakshika was carried out through Bharjana method in Nimbka swarasa for 3 days as mentioned in Rasatarangini.7 After shodhana it was further subjected to Marana with Nimbuka swarasa as marana media as referred to, in Rasatarangini,8 till bhasma siddhi lakshanas were attained upto 10th puta. Sample of Bhasma collected after first puta, fifth puta and tenth puta.
- Organoleptic methods:
Varna (colour) Specific to a specific Bhasma
Sparsha (touch) Soft, Bhasma should not have any course particles that could be detected by touch
Gandha (odour) Should be odourless
Rasa (taste) Should be tasteless
Rupa (appearance) Almost same in all the Bhasma but can vary from Bhasma to Bhasma with very less differences.
B Classical Bhasma Parameters9
Varitaratawa: If a Bhasma floats on water surface it can be regarded as standard one. Surface tension plays important role that is the particles of Bhasma have become so find that they cannot break the surface tension of the water. In the ordinary ways. If the Bhasma attain such state it may be recommended for internal use.
Rekha purnatwa: Here the Bhasma powder is rubbed in between the thumb and fingers and the test is known as Rekha purnatwa. When the Bhasma attain such condition, it could easily enter the furrows of the finger, it is presumed that they may be also absorbed into the system and then the process of Marana may be considered complete.
Nischandratwa: Bhasma should be rub in between the fingers and the thumb and the rubbed portion is examined in Sun rays if any shining particle is seen over the finger then the Bhasma is not good.
Amla parikshya: This test is generally performed for Bhasma containing at least one copper compound as one ingredient. A pinch of prepared Bhasma is mixed with little amount of Dadhi /Curd taken in in a clean and dry petry dish and kept for 48 hours and then observed for any colour change. No colour change of Dadhi /curd shall indicate good quality of Bhasma.
Nisvadutam: The prepared Bhasma shall be tasteless.
Avami: oral intake of Bhasma prepared should not produce any symptoms like nausea / vomiting,
C Physico-Chemical parameters10,11
- Determination of pH value
- Determination of ash value
- Determination of acid insoluble ash
- Determination of water soluble Ash
- Loss on ignition
- Determination of loss on drying
Determination of Iron (Fe)12
Solutions:
? Stannous chloride solutions-Dissolve 5.00 mgSnCl2(A.R) in 25ml hydrochloric acid and dilute to 100 ml (5% solution).
? Mercuric chloride-Saturated solution in purified water.
? Sulphuric acid + orhtphosphoric acid mixture- take 60 ml purified water, add 15 ml sulphuric acid and 15 ml phosphoric acid cool and dilute to1000 ml.
? Diphenyleamine barium sulphonate - Dissolve 0.25 g in 100 ml purified water. 0.1N standard potassium dichromate solution. Dissolve 4.9035 g A.R grade in purified water and dilute to 1000 ml.
Procedure -Take a suitable aliquot from the stock solution in 250 ml conical flask in in duplicate And dilute to about 100 ml with purified water and add 1 - 2 drops of methyl red indicator followed by 122 gram of ammonium chloride and dilute ammonia solution was added till brown precipitate appears and solution with precipitate is boiled for 4-5 minutes then the content cooled and filtered through whatman 41 No. filter paper Wash the residue with hot purified water 4-6 times. Dissolve the residue in dilute hydrochloric acid in 250 ml beaker and make the volume up to 100 ml approx. then boil the solution by adding stannous chloride solution dropwise till solution becomes colourless. Add 1-2 drops stannous chloride in excess and cool the content in purified water. Then add 10 to 15 ml 10% solution of mercuric chloride 25 ml acid mixture and 2-3 drops of diphenyl amine barium sulphonate indicator and purified water if required and titrate against standard potassium dichromate solution, appearance of violet colour shows end point.
Determination of Copper
Procedure: Take suitable aliquot from the the stock solution in a beaker and add approx. 1.0 g Sodium fluoride. Add ammonia solution precipitation occurs and add acetic acid to dissolve the precipitate, boil and cool in water bath. Add approximately 1.0 g potassium iodide and titrate the liberated iodine against 0.1 N sodium sulphate thoisulphate solutions by adding starch solution as indicator in iodine flask. The colour changes from blackish brown to white indicates end point. Calculate copper value against 1 ml of sodium thoisulphate solution titrating against standard 1000-ppm copper solution,
Determination of Sulphur
Solutions: Carbon tetrachloride saturated with Bromine Barium Chloride - 10% solution in purified water
Procedure: Take 0.5- 1.0 g powder sample in 250 ml beaker. Add 10 ml carbon tetra chloride saturated with bromine keep in cold condition in fume chamber overnight and add 10 - 15 ml nitric acid, digest on water bath then add 10 ml hydrochloric acid, digest it to expel NO2 fumes till syrupy mass is obtained. Cool and extract with hydrochloric acid, make volume up to 100 ml, boil and filter through Whatman 40 No. filter paper, wash the residue with hot purified water. Treat the filtrate with ammonia solution for R2O3 precipitation, here R stands for Fe and Al. Filter through whatman 41 No. filter paper in 500 ml beaker, acidify the filtrate with hydrochloric acid and add 20 ml of 10% barium chloride solution. Stir the solution and digest on burner. Allow the precipitate to settle for over night. Filter the precipitate through whatman 42 No. filter paper and wash the precipitate with purified water. Ignite the precipitate in muffle furnace in pre weighed platinum crucible upto 8500C and allowed to cool and weigh. Calculate the weight of sulphur by multiplying weight of precipitate with 0.13734
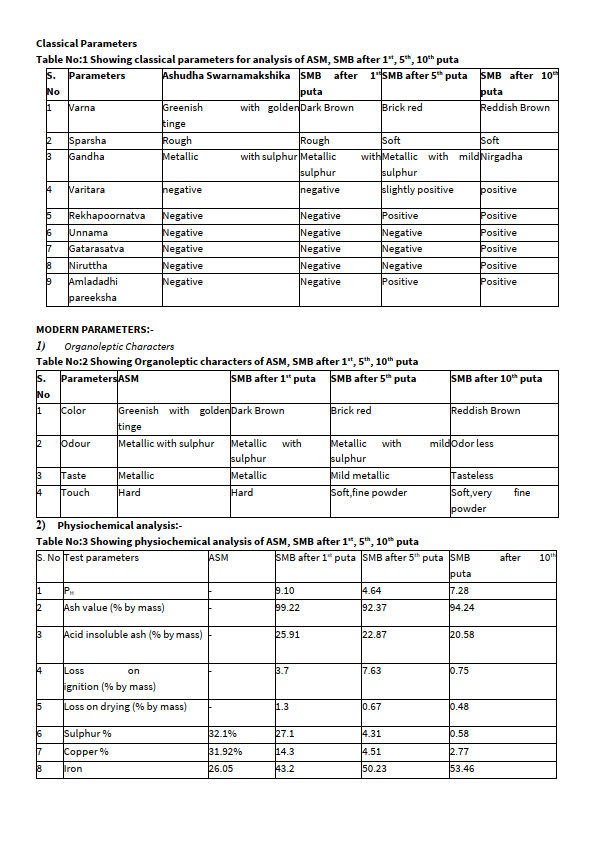
table 1,2,3
Discussion
Discussion on ancient parameters
After trituration with Nimbu Swarasa, colour and consistency of Swarnamakshika paste was rust brown in colour, hard and shiny in nature which turned into dark brown colour with slight decrease in lustre after first puta. This indicates that the process of Marana has influenced its character in bringing out slight loss of lustre but other testing parameters were still not positive. After second and third puta, Swarnamakshik Bhasma attained reddish black colour with loss of lustre to maximum. After fourth and fifth puta, Swarnamakshika Bhasma attained brick red colour with no shiny particles and touch was soft and Rekhapoornata was found positive. After 6th and 7th puta Swarnmakshika Bhasma attained blackish brown colour and Varitara was partially positive. Again in in 8th and 9th puta, the colour of Swarnamakshika Bhasma was slightly reddish brown and sustained all classical parameters except varna. In 10th puta, reddish brown colour was obtained satisfying the colour explained in classics. This indicates that the process of Marana has its influence in bringing about the character like Varna, Nishchandratava, Varitara etc. It is also evident from analytical test that increase in iron oxide is contributing factor in attaining color and loss of lustre. At at the end of fourth puta the powder was fine and almost achieved the quality of Rekhpurnata, but when tasted it was metallic. It was presumed that it may be due to presence of uncalcinated copper, iron hence it was decided to subject the product further for fifth puta. After 5th puta also metallic taste remained and on completion of 7th puta, the metallic taste disappeared. Hence, it could be stated that Swarna makshik Bhasma achieved Gatrasatva at this stage.The Rekhapoornata test was satisfactory after fifth puta, which indicated that particles have attained sukshmata (minuteness). Varitara test was observed partially positive from seventh puta i.e few particles were still sinking in the water and further Putas were continued. Thus after ninth puta, Swarnamakshika Bhasma completely achieved Varitara test. This indicates lightness of the Bhasma. All the parameters were positively passed except Varna, so Marana was extended to tenth puta to obtain all Bhasma Siddhi lakshanas and color of final end product was reddish brown as described in classics.
Modern parameters
(1) Discussion on pH value: pH value of Nimbuka Swarasa Shodhita and Marita Swarnamakshika Bhasma was 7.28 showing that the formulation is alkaline in nature. Normally weakly basic drugs are rapidly absorbed from the intestine. They act rapidly, on oral administration.
(2) Total Ash: Ash value of Swarnamakshik Bhasma was 94.24%.This is according to the standard of Bhasmas.
(3) Acid insoluble Ash: The acid insoluble ash of Swarnamakshik Bhasma was 20.58% which is according to the pharmacopeial standards. It signifies genuinity of the product and suggest that it is best in terms of solubility and absorption.
(4) Loss on drying at 110oC: Loss on drying of Swarnamakshika Bhasma was 0.48 at 110 oC. It is a physical test to notice the percentage of moisture content in sample. Least loss on drying at 110oC, the better will be the drug, rare chances of bacterial or fungal growth. The drug is containing least hygroscopic activity with less chances of contamination of drug.
(5) Loss on ignition: Loss on ignition of Swarnamakshik Bhasma was found to be 0.75% determined by drying the filtrate, and the insoluble ash is determined by rinsing ,drying and ashing the filter paper.
Conclusion
Swarnamakshika is one among Maharasa composed of Copper, Iron and Sulphur. It is considered as a Rasayana agrya and indicated in diseases like kushtha, Prameha, Pandu, anidra etc. Pharmaceutical study revealed that the physical properties like colour, consistency, composition etc. changed during process of Shodhana and Marana. Marana was done until bhasma siddhi lakshanas were attained and it took ten putas. Study of physicochemical parameters revealed increase in percentage of iron and decrease in percentage of copper after Shodhana and subsequent Marana. This may be due to redox reactions. The decrease in percentage of Sulphur from Shodhana and subsequent putas may be due to sublimation of Sulphur.
References
value="
- Sarkar PK, Chaudhary AK. Ayurvedic Bhasma: The most ancient application of nanomedicine. J Sci Ind Res. 2010;69:901–5
- Pandit Kashinath Shastry editor, Agnivesha, pratisamskarana by Charaka and Drudhabala, Charaka Samhita, hindi 6th edition Varanasi, Choukhamba Sanskirt Samsthan, 2000, Chikitsa Sthana, 7th chapter, verse 71-72, page no. 211
- Pandit Kashinath Shastry editor, Agnivesha, pratisamskarana by Charaka and Drudhabala, Charaka Samhita, hindi 6th edition Varanasi, Choukhamba Sanskirt Samsthan, 2000, Chikitsa Sthana, 16th chapter, verse 78-79, page no. 425.
- Sushruta Samhitha with Dalhana teeka, Chikitsasthana edited Yadavji Tikramji Acharaya, Narayan Ramacharya, ChouRhamba Publication, edition 6th year of publication 1997, 14th adhyaya, Shloka 17-18 Page no.456.
- .Atrideva gupta Vidyalankar Astanga Samgraha of Vaghbhata, uttaratantra, Banaras hindu University press, Varanasi, edition 1st 1962, Shloka 49/198-206 page no. 441
- Ambikadatta Shastri Rasaratna Samucchaya of Vaghbhata, published by Choukhamba Bharati Prakashan, Varanasi, 8th edition, 1998 chpater 2nd Sholka 73-88, page no. 48-50
- Shastri K, Rasatarangini of Sadanand Sharma, New delhi, Motilal Baranasi Dass, 2014, Ch. 21 , verse 17, Page No. 525.
- Shastri K, Rasatarangini of Sadanand Sharma, New delhi, Motilal Baranasi Dass, 2014, Ch. 21 , verse 22, Page No. 525.
- Mohaptra, Sudhaldev, and C. B. Jha. "Physicochemical characterization of Ayurvedic bhasma (Swarna makshika bhasma): An approach to standardization." International journal of Ayurveda research 1.2 (2010): 82.
- Honwad S.V A handbook of Standardization of Ayurvedic Formulations, Varanasi, Chaukhambha Orientalia, 2018, Chapter 4, page no 89.
- Kokate C.K, Purohit Gokhale S.B, A Textbook of Pharmacognosy, Pune, Nirali Prakashan, 2002, chapter 1, page no. 58 -60.
- Ayurvedic Formulary of India, 2008, Part I, Volume 7, Page No. 74-75.
"

